Physics Colloquium with David Piston on Quantitative Imaging of Cell Surface Receptor Dynamics
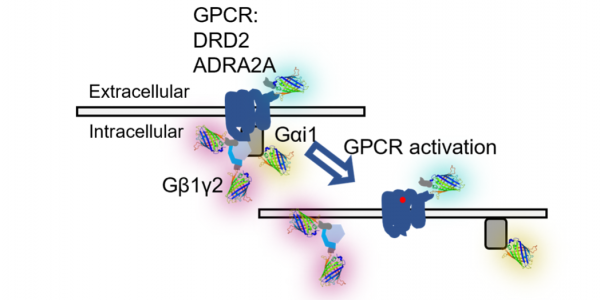
The outer membrane of human cells is decorated with proteins that sense the environment and regulate cell function. One large family of these proteins is the G-protein coupled receptors (GPCR) that are the targets of over 40% of currently marketed pharmaceuticals. In the canonical model, a heterotrimeric G-protein that is pre-coupled to the receptor is released and breaks into pieces upon receptor activation by a signal from outside the cell. One GPCR that we have been interested in is the receptor for the neurotransmitter dopamine, which has been shown to play a role in the regulated release of insulin secreted from pancreatic β-cells and glucagon secreted from the α-cells. The chronic imbalance in insulin/glucagon output is a defining trait of diabetes, and better understanding of the in vivo regulation of these hormones can lead to better treatments for diabetes. We showed that the inhibitory dopamine receptors D3 and D2 are present and active in β-cells, and these along with the stimulatory D1 isoform are present in the α-cells. Towards understanding the complex interplay between the dopamine receptors that are active in the various islet cell types, we have developed multi-color live cell fluorescence imaging approaches to assess potential signaling cascades that differentially regulate insulin and glucagon. As these signaling networks take place within a complex cellular environment with multiple GPCRs acting at once, it is necessary to use highly specific measurement approaches such as photochromic FRET and fluorescence fluctuation spectroscopy (FFS). We have combined hyperspectral two-photon laser scanning imaging FFS and spatial cumulant analysis (SpCA), to measure the interaction dynamics dopamine receptors and G-protein sub-units in islet cell types. Data presented will demonstrate how multicolor SpCA can accurately measure the stoichiometry of receptors, G-proteins, and downstream signaling targets, and reveal molecular aspects underlying the differential control of insulin and glucagon secretion by dopamine.